Overview
- Antibodies are immunologically active circulating proteins that
- are composed of two heavy chains paired with two light chains
- serve as a primary component of humoral immunity
- are produced by B-cells that can further
- differentiate into plasma cells that specialize in secreting antibodies
- mature to make antibodies with higher affinity
- remain dormant as memory cells
- bind antigens from a wide variety of pathogens
- are also known as immunoglobulins (Ig)
- Antibodies are able to fight infections through multiple mechanisms including
- opsonization of the surface of the pathogen leading to
- phagocytosis by innate immune cells like macrophages
- cytotoxicity by triggering release of toxic compounds by innate immune cells
- neutralization of pathogens and viruses by
- blocking interaction of pathogenic proteins with host receptors
- inactivating virulence factors expressed by pathogens
- activation of the complement cascade through the classical pathway
- opsonization of the surface of the pathogen leading to
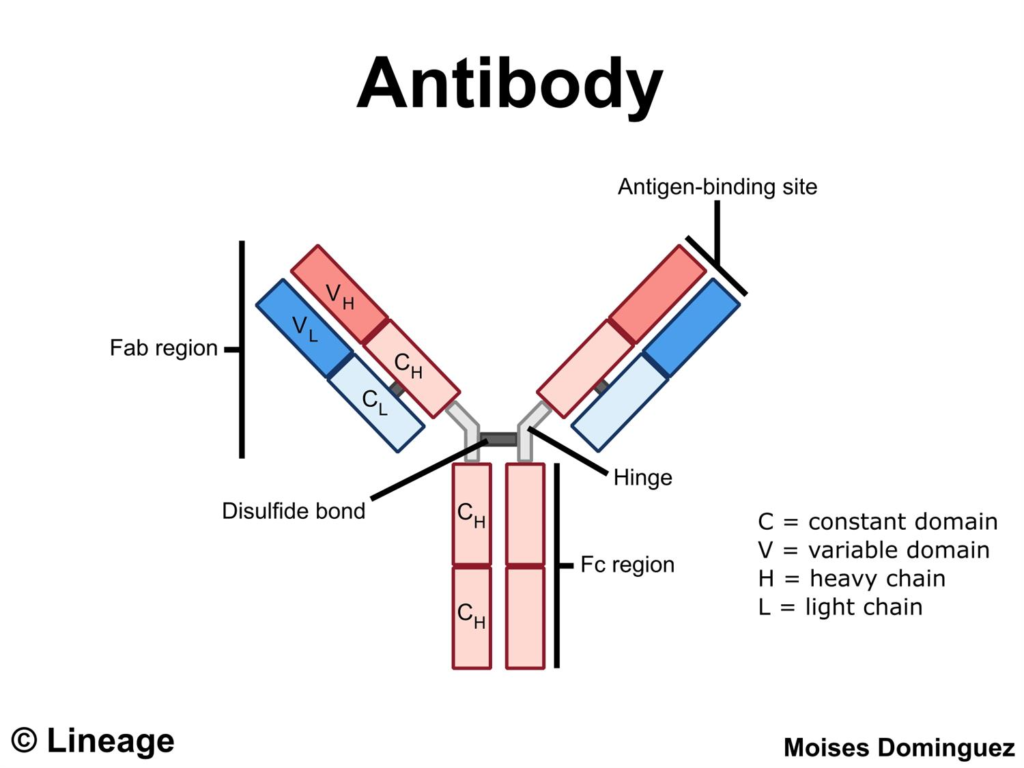
Antibody Structure
- Antibodies are composed of two heavy chains paired with two light chains
- Together these chains create distinct regions of the antibody such as
- the constant fragment (Fc)
- two identical variable antigen binding fragments (Fab)
- These regions differ in both structure and function
Antibody Variation and Diversity
- Antibodies are able to fight an incredible range of infections because of
- idiotype diversity which
- governs what antigens can be recognized by antibodies
- is generated by multiple diversity mechanisms including
- random recombination of VDJ regions of antibody coding regions
- random addition of nucleotides to hypervariable regions by TdT
- random assortment of heavy chains with light chains
- affinity maturation through somatic hypermutation after antigen exposure
- ensures that any moiety can be recognized by the variable region of an antibody
- idiotype diversity which
- isotype diversity through five types of constant regions



