Overview
| Dissolved CO2 | Bicarbonate (HCO3–) | Carbaminohemoglobin |
| ~5-10% of total CO2 content | 70% of total CO2 content | 20-25% of total CO2 contentCO2 bound to N-terminus amino group of hemoglobin (Hb)Stabilizes taut form of Hb → right-shift of O2-Hb dissociation curve (Bohr effect) → favors O2 release |
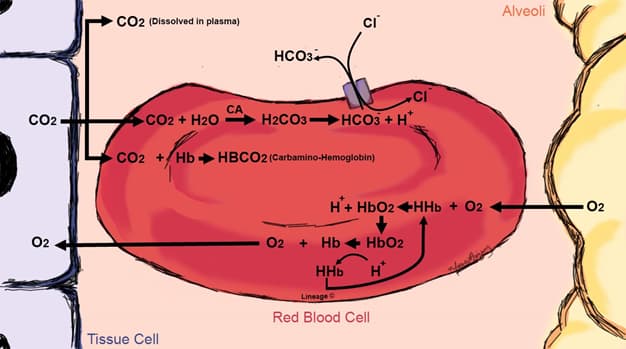
- Transport of CO2 in blood (peripheral tissues → RBC → lungs)
- peripheral tissues
- metabolically active tissues produce CO2, which diffuses across the cell membrane and eventually into the RBC at the level of the capllaries
- RBC
- carbaminohemoglobin
- ↑ pCO2 is produced in peripheral tissues
- this facilitates CO2 binding to Hb, forming carbaminohemoglobin (CO2-Hb)
- in turn, the Taut (T) state of Hb is stabilized
- Bohr effect
- enhanced release of O2 in the presence of low pH or increased pCO2
- CO2-Hb → ↓ Hb affinity for O2 → ↑ O2 unloading into tissues
- H+ produced by converting CO2 + H2O to HCO3– + H+ via carbonic anhydrase (CA) which contributes in lowering the pH
- protonated Hb stabilizes the T state, facilitating O2 release into tissues
- (oxyhemoglobin) HbO2 + H+ ↔ HbH + O2 (deoxyhemoglobin)
- enhanced release of O2 in the presence of low pH or increased pCO2
- Haldane effect
- at low pO2 levels, the carrying capacity of CO2 increases
- in other words, there is an increase affinity for CO2 when there is less O2 bound to hemoglobin
- at low pO2 levels, the carrying capacity of CO2 increases
- Bohr effect
- ↑ pCO2 is produced in peripheral tissues
- bicarbonate (HCO3–)
- CA reversibly catalyzes the formation of HCO3– from the hydration of CO2
- this reaction is reversible, thus CA is also involved in the dehydration of H2CO3
- CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
- the produced H+ remains in the RBC, where it is buffered by deoxyhemoglobin
- deoxyhemoglobin is a better H+ buffer than oxyhemoglobin
- CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
- the produced HCO3– is transported into the plasma via a chloride-bicarbonate (Cl-HCO3) exchanger (AE1 or band three protein)
- this describes the chloride (or Hamburger) shift
- carbaminohemoglobin
- lung
- the reactions mentioned above occur in reverse inside the lung
- carbaminohemoglobin
- Bohr effect
- in lungs (low PCO2), CO2 dissociates from hemoglobin and stabilizes high O2 affinity Relaxed (R) state
- hemoglobin-oxygen dissociation curve left shifts → ↑ hemoglobin affinity for O2 → ↑ O2 loading
- in lungs (low PCO2), CO2 dissociates from hemoglobin and stabilizes high O2 affinity Relaxed (R) state
- Haldane effect
- O2 loading → ↓ hemoglobin affinity for CO2 → ↑ CO2 unloading
- Bohr effect
- bicarbonate
- HCO3– is exchanged for Cl– (chloride shift) across RBC membranes
- HCO3– enters RBCs
- Haldane effect
- O2 loading → ↓ hemoglobin affinity for H+→ ↑ H+ unloading
- H+ + HCO3– → H2CO3 → CO2 + H2O
- ↑ H+ drives equilibrium reactions to right (CO2 formation).
- HCO3– is exchanged for Cl– (chloride shift) across RBC membranes
- carbaminohemoglobin
- the reactions mentioned above occur in reverse inside the lung
- peripheral tissues



