Overview – Pharmacokinetics
Pharmacokinetics is the study of how drugs are absorbed, distributed, metabolized, and eliminated by the body. It involves understanding the time course and fate of drugs within the body, including their absorption into the bloodstream, distribution to tissues, metabolism by enzymes, and elimination through various routes.
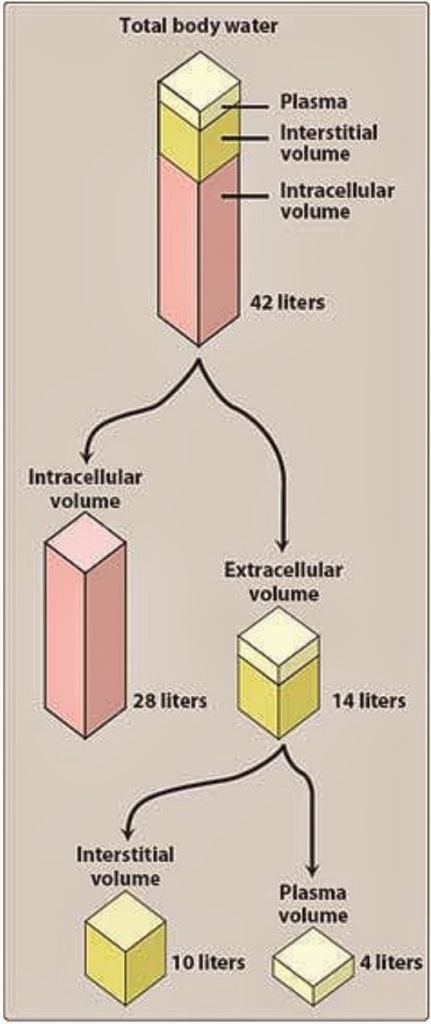
Volume of Distribution (Vd)
- Volume of distribution (Vd) relates amount of drug in body to plasma concentration
- Vd = (amount of drug in the body) / (plasma drug concentration)
- Vd is changed in disease states that decrease plasma proteins
- a decrease in plasma proteins decreases binding of drug to plasma proteins
- ↑ Vd of drugs
- e.g., liver disease
- via ↓ protein synthesis
- e.g., kidney disease
- via urinary protein loss
- a decrease in plasma proteins decreases binding of drug to plasma proteins
- Vd increased in disease states that increase total body water
- Vd predicts drug distribution in body
- low Vd drugs
- 4-8 L
- drugs distribute in vascular compartment (blood) and bind plasma proteins
- drugs are large and/or charged molecules
- medium Vd drugs
- drugs distribute in extracellular compartment and/or total body water
- drugs are small, hydrophilic molecules that do not bind plasma proteins
- high Vd drugs
- > body weight
- drugs distribute in all tissues
- low Vd drugs
- drugs are small, lipophilic molecules that bind strongly to extravascular proteins
Clearance (CL)
- CL = (rate of elimination of drug) / (plasma drug concentration) = Vd * Ke
- Ke = elimination constant
- CL relates the rate of elimination to the plasma concentration
Half-Life (t1/2)
- t1/2 = (0.7 * Vd)/CL
- half-life is time required for amount of drug to fall to 50% of an earlier measurement during elimination or during constant infusion
- for drugs eliminated by first-order kinetics, half-life is constant regardless of concentration
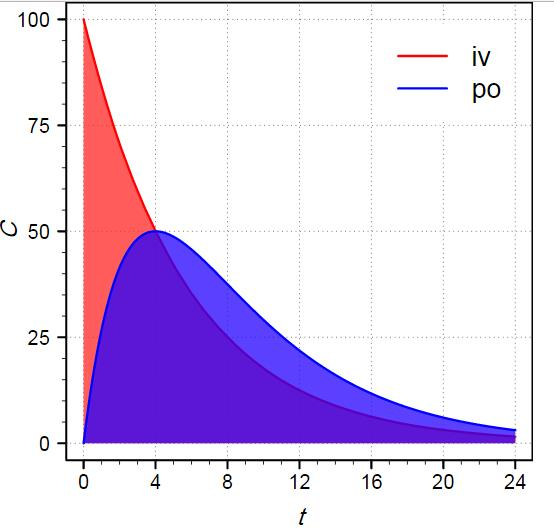
Bioavailability (F)
- bioavailability is the fraction of administered dose that reaches systemic circulation
- bioavailability is defined as unity, or 100%, in case of IV administration
- bioavailability of drug administered by other routes is generally reduced by incomplete absorption, first-pass metabolism, and any distribution into other tissues that occurs before the drug enters systemic circulation
- e.g., orally, F = percent that is absorbed and survives first-pass metabolism in liver
- Calculation of bioavailabillity (F) = 100% * (AUC-oral * Dose-IV) / (AUC-IV * Dose-oral), where AUC is the area under the curve of a pharmacokinetic plasma concentration versus time plot
- delayed release formulations will have slower rise and lower peak compared with rapid release formulations
Key Processes in Pharmacokinetics
- Absorption: Absorption refers to the process by which a drug enters the bloodstream from its site of administration. The route of administration plays a significant role in determining the rate and extent of drug absorption. Common routes of administration include oral (by mouth), intravenous (directly into the veins), intramuscular (into the muscle), subcutaneous (under the skin), and transdermal (through the skin).
- Distribution: After absorption, drugs are distributed throughout the body via the bloodstream. Distribution depends on various factors, including blood flow to different organs, drug solubility, and binding to plasma proteins or tissues. Some drugs may also cross biological barriers, such as the blood-brain barrier, placental barrier, or blood-milk barrier, to reach their target sites or exert systemic effects.
- Metabolism: Drug metabolism involves the enzymatic conversion of drugs into metabolites, which are usually more polar and easier to eliminate. The liver is the primary organ responsible for drug metabolism, although other organs like the intestines and lungs can also metabolize drugs to some extent. Enzymes involved in drug metabolism are primarily from the cytochrome P450 (CYP) family. Metabolism can either activate or inactivate drugs, and it plays a crucial role in determining a drug’s duration of action and potential interactions with other drugs.
- Elimination: Drug elimination involves the removal of drugs and their metabolites from the body. The primary routes of elimination are renal (through the kidneys) and hepatic (through the liver). Renal elimination occurs through filtration in the kidneys followed by excretion in the urine. Hepatic elimination involves the excretion of drugs and metabolites into the bile, which can then be eliminated in the feces. Other routes of elimination include excretion in sweat, saliva, breast milk, and exhaled air.
- Pharmacokinetic Parameters: Pharmacokinetics uses various parameters to quantitatively describe the processes mentioned above. Some commonly used parameters include:
- Bioavailability: The fraction of an administered dose that reaches systemic circulation unchanged, typically expressed as a percentage for oral drugs.
- Clearance: The rate at which a drug is removed from the body, often expressed as milliliters per minute (mL/min) or liters per hour (L/h).
- Half-life: The time it takes for the concentration of a drug in the body to decrease by half, indicative of the drug’s elimination rate.
- Volume of distribution: A theoretical volume that represents the apparent space in the body where a drug is distributed, relative to its concentration in the plasma.
Understanding the pharmacokinetic properties of drugs is crucial for determining optimal dosing regimens, assessing drug-drug interactions, and predicting drug concentrations in different patient populations. It helps healthcare professionals ensure safe and effective use of medications in clinical practice.
Studies – Pharmacokinetics
Pharmacokinetics studies are conducted to understand the absorption, distribution, metabolism, and elimination of drugs in the body. These studies provide valuable information about a drug’s pharmacokinetic profile, which is essential for determining optimal dosing regimens, predicting drug-drug interactions, and assessing the safety and efficacy of medications. Here are some common types of pharmacokinetics studies:
- Bioavailability Studies: Bioavailability studies assess the extent and rate at which a drug is absorbed into the systemic circulation. They compare different formulations or routes of drug administration, such as oral, intravenous, or transdermal, to determine the bioavailability of a drug. These studies help evaluate the effectiveness of drug delivery systems and guide dosing recommendations.
- Drug Distribution Studies: Distribution studies investigate how drugs are distributed throughout the body after systemic absorption. They examine drug concentrations in different tissues and organs over time to determine the drug’s volume of distribution and tissue-specific distribution patterns. These studies provide insights into the drug’s tissue targeting and potential accumulation in specific organs.
- Metabolism Studies: Metabolism studies focus on the enzymatic conversion of drugs into metabolites and the pathways involved. They assess the metabolic stability of a drug, identify the enzymes responsible for its metabolism (such as cytochrome P450 enzymes), and evaluate the potential for drug-drug interactions affecting metabolism. Metabolism studies also investigate the impact of genetic polymorphisms on drug metabolism.
- Clearance Studies: Clearance studies examine the rate at which drugs are eliminated from the body. They measure drug concentrations in plasma or urine over time to estimate clearance values, which reflect the efficiency of drug elimination. Clearance studies can provide valuable information on the influence of renal or hepatic function on drug elimination and guide dosing adjustments in patients with impaired organ function.
- Pharmacokinetic Modeling and Simulation: Pharmacokinetic modeling and simulation involve the use of mathematical models to describe drug concentration-time profiles and predict pharmacokinetic behavior. These studies integrate data from multiple pharmacokinetic studies and incorporate patient-specific factors to generate individualized pharmacokinetic predictions. Pharmacokinetic modeling and simulation help optimize dosing regimens, predict drug concentrations, and assess the impact of various factors on drug exposure.
- Clinical Pharmacokinetic Studies: Clinical pharmacokinetic studies involve evaluating pharmacokinetics in patient populations to assess drug safety, efficacy, and dosing guidelines. These studies often include patient subgroups with different characteristics, such as age, renal or hepatic impairment, or concomitant medications. Clinical pharmacokinetic studies provide important information for determining appropriate dosing strategies in specific patient populations.
Pharmacokinetics studies are typically conducted during drug development, in preclinical and clinical phases, to characterize a drug’s pharmacokinetic properties. They contribute to the understanding of a drug’s behavior in the body, guide dosing recommendations, and support evidence-based decision-making in clinical practice.
Check out USMLE Step 1 Mastery: Comprehensive Course and Lecture Notes.


