Overview
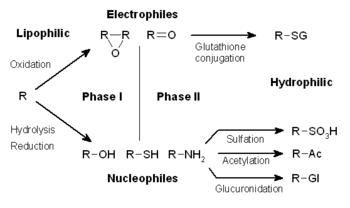
- Phase I metabolism
- oxidation (via cytochrome P450), reduction, and hydrolysis reactions
- phase I reactions convert a parent drug to more polar (water soluble) active metabolites by unmasking or inserting a polar functional group (-OH, -SH, -NH2)
- geriatric patients have decreased phase I metabolism
- drugs metabolized via phase I reactions have longer half-lives
- geriatric patients metabolism drugs by phase II reactions
- Phase II metabolism
- glucuronidation, acetylation, and sulfation reactions
- “conjugation reactions” that increase water solubility of drug with a polar moiety
- glucuronate, acetate, and sulfate, respectively
- phase II reactions convert a parent drug to more polar (water soluble) inactive metabolites by conjugation of subgroups to -OH, -SH, -NH2 functional groups on drug
- drugs metabolized via phase II reactions are renally excreted
- patients deficient in acetylation capacity (slow acetylators) may have prolonged or toxic responses to normal doses of certain drugs because of decreased rates of metabolism
- “conjugation reactions” that increase water solubility of drug with a polar moiety
- glucuronidation, acetylation, and sulfation reactions
What is Phase I Metabolism?
Phase I metabolism refers to the initial metabolic reactions that occur in the body to transform drugs, toxins, and other foreign substances into more polar (water-soluble) compounds. This phase involves various enzymatic reactions that introduce or expose functional groups on the molecule, making it more amenable to subsequent modifications in Phase II metabolism or easier for elimination from the body.
The primary goal of Phase I metabolism is to increase the polarity and water solubility of the compound, facilitating its elimination through urine or bile. The reactions involved in Phase I metabolism include oxidation, reduction, and hydrolysis. The enzymes responsible for these reactions are primarily found in the liver, although they can also be present in other tissues.
There are several major enzyme families involved in Phase I metabolism, including:
- Cytochrome P450 (CYP) enzymes: This is the most prominent family of enzymes involved in Phase I metabolism. They play a crucial role in oxidizing various substances by introducing or unmasking functional groups like hydroxyl (-OH), carboxyl (-COOH), or amine (-NH2) moieties.
- Flavin-containing monooxygenases (FMO): FMO enzymes catalyze oxidative reactions similar to cytochrome P450 enzymes but are involved in the metabolism of specific substrates, including some drugs and dietary compounds.
- Alcohol and aldehyde dehydrogenases: These enzymes are involved in the oxidation of alcohols and aldehydes, converting them into more polar carboxylic acids.
- Esterases and amidases: These enzymes catalyze the hydrolysis of esters and amides, breaking them down into their corresponding acids and alcohols or amines.
Phase I metabolism can result in the production of metabolites that may have different pharmacological activities compared to the parent compound. These metabolites can be either inactive, more active, or even toxic. Moreover, some compounds may undergo bioactivation during Phase I metabolism, forming reactive intermediates that can cause adverse effects or contribute to toxicity.
After Phase I metabolism, the metabolites may undergo Phase II metabolism, which involves conjugation reactions, such as glucuronidation, sulfation, acetylation, methylation, and glutathione conjugation. These Phase II reactions further increase the water solubility of the metabolites, aiding in their elimination from the body.
Overall, Phase I metabolism represents the initial step in the biotransformation of drugs and foreign compounds, helping to prepare them for subsequent metabolic processes and elimination from the body.
What is Phase II Metabolism?
Phase II metabolism, also known as conjugation or synthetic metabolism, is the second phase of drug and xenobiotic metabolism that follows Phase I metabolism. In Phase II metabolism, the transformed Phase I metabolites or the unchanged parent compound undergo conjugation reactions with endogenous molecules to form more polar and water-soluble compounds. These conjugation reactions increase the compound’s stability, facilitate its excretion, and reduce its toxicity.
The conjugation reactions involved in Phase II metabolism typically involve the transfer of a functional group from an endogenous molecule to the drug or xenobiotic molecule. The most common Phase II reactions include:
- Glucuronidation: Glucuronic acid is attached to the substrate molecule by UDP-glucuronosyltransferase (UGT) enzymes, forming a glucuronide conjugate. Glucuronidation is one of the most common Phase II reactions and results in highly polar and water-soluble compounds.
- Sulfation: Sulfate groups are added to the substrate molecule by sulfotransferase enzymes, forming a sulfate conjugate. Sulfation commonly occurs with phenols and amines.
- Methylation: Methyl groups are transferred to the substrate molecule by methyltransferase enzymes, resulting in a methylated conjugate. Methylation reactions often occur with compounds containing hydroxyl (-OH), amine (-NH2), or thiol (-SH) functional groups.
- Acetylation: Acetyl groups are added to the substrate molecule by acetyltransferase enzymes, forming an acetylated conjugate. Acetylation commonly occurs with arylamines and hydrazines.
- Glutathione Conjugation: Glutathione, a tripeptide composed of glutamate, cysteine, and glycine, is attached to the substrate molecule by glutathione S-transferase (GST) enzymes. Glutathione conjugation plays a significant role in detoxifying reactive electrophilic compounds.
These Phase II conjugation reactions result in the formation of larger and more polar molecules that are generally less biologically active and more readily excreted from the body. The conjugates formed during Phase II metabolism are often less lipophilic than the parent compounds or Phase I metabolites, making them more soluble in water and easier to eliminate through urine or bile.
What are the Similarities Between Phase I and Phase II Metabolism?
Phase I and Phase II metabolism are both involved in the biotransformation of drugs, xenobiotics, and endogenous compounds in the body. While they have distinct characteristics and reactions, there are several similarities between Phase I and Phase II metabolism:
- Biotransformation: Both Phase I and Phase II metabolism involve chemical transformations of compounds to facilitate their elimination from the body. They are part of the overall process of drug metabolism and detoxification.
- Metabolite Formation: Both phases result in the formation of metabolites. Phase I metabolism generates primary metabolites, which are often intermediate products with increased polarity compared to the parent compound. Phase II metabolism then further modifies these metabolites, resulting in the formation of conjugated metabolites that are more water-soluble and suitable for excretion.
- Enzymatic Reactions: Both phases rely on enzymatic reactions catalyzed by specific enzymes. Phase I metabolism involves enzymes such as cytochrome P450 (CYP) enzymes, flavin-containing monooxygenases (FMO), alcohol dehydrogenases, and esterases, among others. Phase II metabolism involves enzymes such as UDP-glucuronosyltransferases (UGT), sulfotransferases, methyltransferases, acetyltransferases, and glutathione S-transferases (GST).
- Transformation of Lipophilic Compounds: Both phases aim to increase the water solubility and polarity of lipophilic compounds. Phase I metabolism introduces or unmasks functional groups on the compound, while Phase II metabolism adds endogenous molecules to the compound to enhance its hydrophilicity.
- Contribution to Drug Clearance: Both Phase I and Phase II metabolism play important roles in the overall clearance of drugs and xenobiotics from the body. By increasing the hydrophilicity of compounds, both phases facilitate their elimination through urine or bile.
- Interplay and Sequential Nature: Phase I and Phase II metabolism are sequential and interconnected processes. Phase II metabolism often occurs after Phase I metabolism, with Phase I metabolites serving as substrates for Phase II conjugation reactions. The combined actions of both phases result in the ultimate transformation and elimination of compounds from the body.
It’s important to note that while there are similarities between Phase I and Phase II metabolism, there are also distinct differences in the types of reactions, enzymes involved, and the nature of the metabolites formed. Understanding these differences is crucial for comprehending the overall process of drug metabolism and its implications for drug efficacy, toxicity, and elimination.
What is the Difference Between Phase I and Phase II Metabolism?
Phase I and Phase II metabolism are two distinct phases of drug and xenobiotic metabolism in the body. They differ in terms of their reactions, enzymes involved, and the nature of the metabolites formed.
Here are the key differences between Phase I and Phase II metabolism:
- Reactions:
- Phase I Metabolism: Phase I reactions involve oxidative, reductive, and hydrolytic transformations of the parent compound or drug. These reactions introduce or unmask functional groups (e.g., hydroxyl, carboxyl, amino) on the molecule, often resulting in the formation of intermediate metabolites. Phase I reactions can generate more polar or less polar compounds depending on the specific reaction.
- Phase II Metabolism: Phase II reactions involve conjugation reactions, where the transformed Phase I metabolites or the unchanged parent compound are conjugated with endogenous molecules. The conjugation reactions transfer functional groups (e.g., glucuronic acid, sulfate, methyl, acetyl) from endogenous molecules to the drug or metabolite, resulting in the formation of highly polar and water-soluble conjugates.
- Enzymes:
- Phase I Metabolism: Phase I reactions are primarily catalyzed by enzymes such as cytochrome P450 (CYP) enzymes and flavin-containing monooxygenases (FMO), along with other enzymes such as alcohol dehydrogenases and esterases. These enzymes are responsible for introducing or modifying functional groups on the compound.
- Phase II Metabolism: Phase II reactions are catalyzed by enzymes specific to each conjugation reaction. Examples include UDP-glucuronosyltransferases (UGT) for glucuronidation, sulfotransferases for sulfation, methyltransferases for methylation, acetyltransferases for acetylation, and glutathione S-transferases (GST) for glutathione conjugation.
- Nature of Metabolites:
- Phase I Metabolism: Phase I metabolites can be intermediate products with increased or decreased polarity compared to the parent compound. They may retain some pharmacological activity and can sometimes be more toxic than the parent compound. Phase I metabolites often serve as substrates for Phase II conjugation reactions.
- Phase II Metabolism: Phase II metabolites are highly polar and water-soluble conjugates formed by the addition of endogenous molecules. These conjugates are typically less pharmacologically active and less toxic than the parent compound or Phase I metabolites. The conjugation reactions significantly increase the hydrophilicity of the metabolites, facilitating their elimination from the body.
- Timing and Sequential Nature:
- Phase I Metabolism: Phase I metabolism often occurs prior to Phase II metabolism, although both phases can occur concurrently. Phase I reactions are usually the initial steps in the biotransformation process, generating metabolites that can serve as substrates for Phase II conjugation reactions.
- Phase II Metabolism: Phase II metabolism typically follows Phase I metabolism, using the transformed metabolites or the parent compound as substrates for conjugation reactions. Phase II reactions further modify the metabolites, enhancing their water solubility and facilitating their elimination.
Understanding the differences between Phase I and Phase II metabolism is crucial for assessing the overall metabolic fate of drugs and xenobiotics, predicting their pharmacological activities and toxicity, and optimizing drug therapy. The sequential and complementary actions of both phases contribute to the efficient elimination of compounds from the body.
Check out USMLE Success Strategy: Personalized Consultation and Study Plan Development.



