Introduction – Pyruvate Dehydrogenase Complex
- Function
- catalyze conversion of pyruvate to acetyl-CoA
- net reaction
- pyruvate + NAD+ + CoA → acetyl-CoA + CO2 + NADH
- irreversible
- Structure
- 3 enzymes that require 5 cofactors
- thiamine pyrophosphate (B1)
- FAD (B2)
- NAD (B3)
- CoA (B5)
- lipoic acid
- similar to α-ketoglutarate dehydrogenase complex
- 3 enzymes that require 5 cofactors
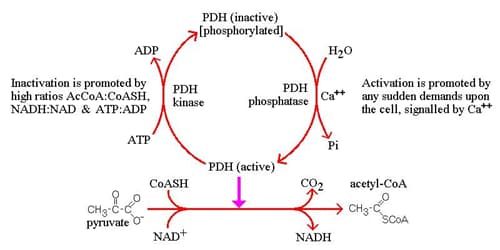
- Regulation
- activators of pyruvate dehydrogenase
- ↓ in energy status of the cell
- ↑ NAD+/NADH ratio
- ↑ ADP
- ↑ exercise
- ↑ Ca2+
- ↑ insulin
- ↓ in energy status of the cell
- inhibited by acetyl-CoA
- activators of pyruvate dehydrogenase
- Clinical relevance
Overview – Pyruvate Dehydrogenase Complex
Pyruvate dehydrogenase complex (PDC) is a multi-enzyme complex found in the mitochondria of eukaryotic cells and in the cytoplasm of some bacteria. The PDC catalyzes an important step in cellular respiration, the conversion of pyruvate to acetyl-CoA, which is a critical molecule for the production of ATP through the tricarboxylic acid cycle (TCA).
The PDC is composed of three main enzymes: pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2), and dihydrolipoamide dehydrogenase (E3). Each enzyme performs a specific step in the conversion of pyruvate to acetyl-CoA.
Structure
The E1 component of the complex consists of multiple copies of pyruvate dehydrogenase (PDH), which is responsible for the decarboxylation of pyruvate. PDH is composed of two subunits, referred to as α and β, and each subunit contains several domains. The α subunit contains two domains, one of which binds to the coenzyme thiamine pyrophosphate (TPP), which is essential for the catalytic activity of PDH. The β subunit contains two domains, one of which binds to pyruvate, and the other binds to the α subunit. The α and β subunits of PDH are arranged in a cubic structure, forming a central core to which the other components of the complex are attached.
The E2 component of the complex is composed of dihydrolipoamide acetyltransferase (DLAT), which contains multiple copies of a single subunit. The subunit contains a lipoic acid prosthetic group that is essential for the catalytic activity of DLAT. The lipoic acid prosthetic group is covalently attached to a lysine residue in the subunit, and it undergoes a series of redox reactions during the conversion of pyruvate to acetyl-CoA.
The E3 component of the complex is composed of dihydrolipoamide dehydrogenase (DLD), which also contains multiple copies of a single subunit. DLD is responsible for the regeneration of the lipoic acid prosthetic group of DLAT, which is necessary for the catalytic cycle of the complex.
Types of Pyruvate Dehydrogenase Complex
The pyruvate dehydrogenase complex (PDC) is an enzyme complex that plays a crucial role in cellular respiration by catalyzing the conversion of pyruvate to acetyl-CoA. There are three types of PDC, which differ in their composition and regulation:
- E1-only PDC: This type of PDC contains only the E1 component of the complex, which is responsible for decarboxylating pyruvate to produce acetyl-CoA. E1-only PDC is found in some bacteria and archaea.
- Classical PDC: This type of PDC is found in eukaryotes and some bacteria and consists of three components: E1, E2, and E3. E1 decarboxylates pyruvate, E2 transfers the resulting acetyl group to CoA to form acetyl-CoA, and E3 regenerates the oxidized form of the enzyme that accepts electrons from E2 during catalysis.
- Alternative PDC: This type of PDC is found in some bacteria and archaea and consists of a modified form of E2 that can perform the functions of both E2 and E3. This allows the complex to function without a separate E3 component, making it more streamlined than classical PDC.
Studies – Pyruvate Dehydrogenase Complex
The pyruvate dehydrogenase complex (PDC) has been the subject of numerous studies aimed at understanding its structure, function, and regulation. Some of the key studies on PDC are:
- Structure determination: In 1985, the first high-resolution crystal structure of PDC was determined using X-ray crystallography. This study revealed the overall architecture of the complex and provided insights into its catalytic mechanism.
- Regulation of PDC activity: PDC activity is regulated by a variety of factors, including phosphorylation, allosteric regulation, and the availability of coenzymes. Studies have shown that phosphorylation of the E1 subunit by pyruvate dehydrogenase kinase (PDK) inhibits PDC activity, while dephosphorylation by pyruvate dehydrogenase phosphatase (PDP) activates the complex.
- Role in disease: Mutations in genes encoding PDC subunits have been associated with a range of diseases, including congenital lactic acidosis, Leigh syndrome, and Parkinson’s disease. Studies have sought to understand the molecular mechanisms underlying these diseases and to develop therapeutic approaches based on modulating PDC activity.
- Engineering PDC for biotechnology applications: PDC has been engineered for use in a variety of biotechnology applications, including the production of biofuels and other value-added chemicals. Studies have focused on optimizing PDC activity and stability under a range of conditions, as well as developing new PDC variants with improved catalytic properties.
Overall, studies on Pyruvate Dehydrogenase Complex have provided important insights into the fundamental mechanisms of cellular respiration, as well as potential applications in biotechnology and human health.
Complications – Pyruvate Dehydrogenase Complex
Complications associated with the pyruvate dehydrogenase complex (PDC) can arise from genetic mutations, environmental factors, or other underlying health conditions. Some of the complications associated with PDC dysfunction include:
- Metabolic disorders: Defects in PDC function can lead to a range of metabolic disorders, including congenital lactic acidosis, Leigh syndrome, and pyruvate dehydrogenase deficiency. These disorders are characterized by a buildup of lactate and other toxic metabolites, which can lead to muscle weakness, developmental delays, and other symptoms.
- Neurological disorders: PDC dysfunction has been implicated in a number of neurological disorders, including Parkinson’s disease, Alzheimer’s disease, and schizophrenia. Studies have shown that PDC dysfunction can impair energy metabolism in the brain, leading to neurodegeneration and cognitive deficits.
- Cardiovascular disease: PDC dysfunction has been linked to an increased risk of cardiovascular disease, including heart failure and ischemic heart disease. Studies have shown that PDC dysfunction can lead to oxidative stress, inflammation, and endothelial dysfunction, all of which contribute to the development of cardiovascular disease.
- Cancer: Pyruvate Dehydrogenase Complex dysfunction has been implicated in the development and progression of certain types of cancer, including breast cancer, prostate cancer, and glioblastoma. Studies have shown that PDC dysfunction can alter cellular metabolism and promote tumor growth and survival.
Check out Interview Preparation, CV and Personal Statement Editing – The Finale.



